Econ 1101—Reading 6
International Application: Intellectual
Property Protection and the Global Pharmaceutical Industry
By Thomas J. Holmes, Dept. of
Economics,
Revised August 28, 2010
for Econ 1101
This
Case Study begins by discussing the general economics of the global
pharmaceutical industry. Part 2
discusses a numerical example of drug pricing with patents. Part 3 analyzes the incentive for research
and development in the example. Part 4
wraps things up by mentioning recent international agreements concerning intellectual
property protection for the drug industry.
Part 1. A Brief Discussion of the Economics of the
Pharmaceutical Industry
It
is expensive to generate and test new drugs.
A study by the Congressional Budget Office report (CBO
(2006)) reports American pharmaceutical firms spend tens of billions of
dollars per year in research and development expenditures. (It reports a range of 20 to 50 billion
dollars that depends on whether costs of testing are included, whether overseas
affiliated are included, and so on.) Drug companies are willing to make these
investments because of the high profits that can be generated when a successful
new drug is generated and the company is awarded a patent. A patent grants the
company a monopoly on the drug. In the
United States, a patent lasts for 20 years.
When a patent expires, other companies are free to enter and market the
generic equivalent of a drug. When a
patent expires and the branded drug is forced to compete with generics, branded
drug revenues typically drop precipitously.
For
example, consider the drug Zocor, sold by Merck. The patent expired on June 23,
2006. (See this New York
Times article from that date for a discussion.) Figure 1 below shows U.S. quarterly sales of
the drug. Before patent expiration,
Merck was averaging sales of $800 million per quarter. As can be seen in the figure below, sales
immediately dropped to less than $200 million a quarter, a 75 percent
decline. Sales continued to deteriorate
beyond 2006 as generics ate away more of Zocor’s market share.
Figure 1
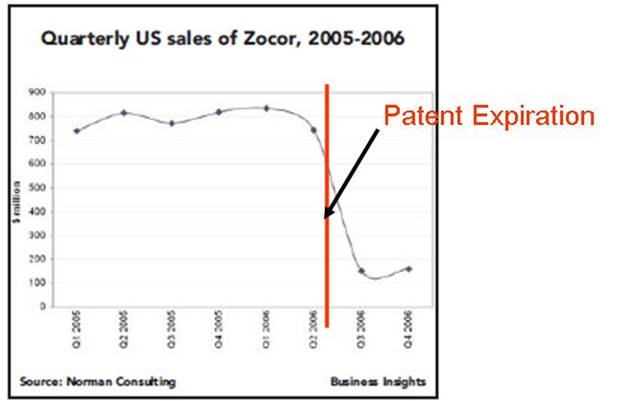
Source:
Norman Consulting: Patent Production Strategies
Drug
companies tend to make a disproporationate share of their profits from sales to
the United States. The same New York
Times article referenced above notes that of Zocor’s worldwide sales of $4.4
billion in 2005, a remarkable $3.1 billion were sales in the United
States. So even though the United States
represents only 5 percent of the world’s population and 25 percent of the
world’s income, it accounted for almost 75 percent of Zocor’s revenue that
year. This pattern holds more
generally. Mark McCllenan, former
commissioner of the Food and Drug Administration, has noted (see
this 2003 speech) that the United States accounts for about half of
worldwide drug company revenues, while Germany contributes only around 5
percent. Germany is a rich country
about one quarter of the size of the U.S., in both population and income. Yet it pays about one tenth as much in drug
expenditures. A similar story is true
about Canada. The Canadian government
bargains with drug companies to get the prices down. It is well known that drug prices are lower
in Canada than in the United States.
Residents who live in Minnesota have an incentive to cross the border
into Canada to buy drugs at a substantially reduced price.
Part 2: Patent and Drug
Pricing
Let’s
work through a numerical example to understand drug pricing with and without
patents. Suppose consumers throughout
the world have the same demand curve for a new drug, called wigitor and this is illustrated in
Figure 2 below. Suppose it is produced
by Econland Big Pharma, Inc. (If there really was such drug I might try to
get a prescription for it!) The drug is
prescribed to people afflicted with the dreaded disease of economyosis. Demand is
graphed on the basis of per person afflicted by the disease, so we can contrast
what happens in different countries that vary in population. For demand, we use the usual example where
the vertical and horizontal intercepts equal 10. The marginal cost is constant equal to $2
(this is also the average variable cost).
The $2 represents the production cost of making each wigitor pill. This is distinct from the fixed cost to
research and develop the drug (discussed below). We consider three possible cases for
pricing.
Case A: Econland Big
Pharma Has a Patent and Faces No Price Regulation
Suppose
first that Econland Big Pharma has a patent on wigitor. Suppose also that it faces no price
regulation. It can then act like
monopolist to maximize profit. Think of
this case as representing the United States.
(Well, the U.S. except for the Veterans Administration (VA). The VA
actually does bargain with the drug companies and obtains low drug
prices just like in
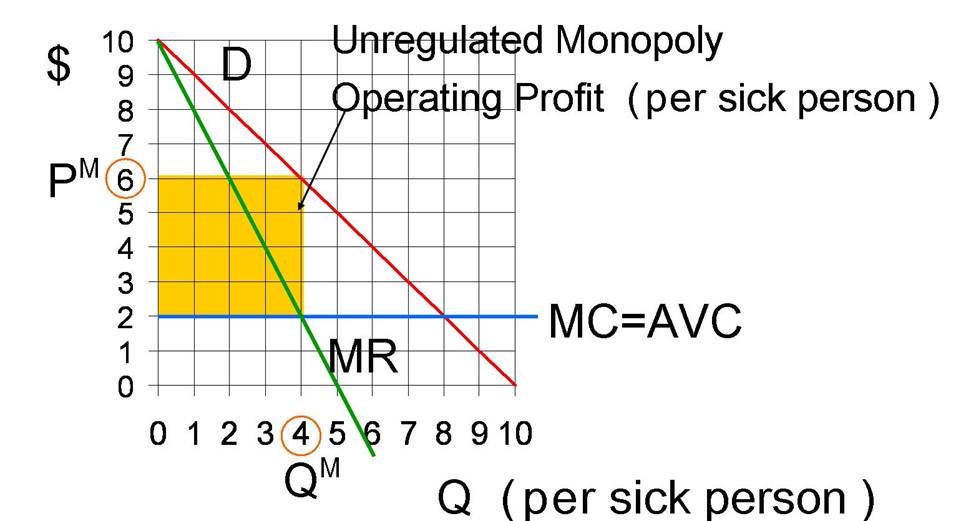
The
monopoly quantity (per sick person) equals 4 doses of the drug, because that is
where marginal revenue equals marginal cost of $2. At the monopoly quantity of 4 doses, the
monopoly price equals $6 per dose. This
yields a revenue per sick person equal to $6×4=$24. The variable cost to produce this amount per
sick person equals $2×4=$8. Define operating profit to be the difference
between revenues and variable costs.
Operating profit does not take into account any fixed cost of research
and development. Operating profit equals
$16 (=$24-$8) and is illustrated by the yellow box below.
Figure 2: Unregulated
Monopoly Price and Quantity for Wigitor (on a per sick person basis)
Case B: No Intellectual
Property Protection
Suppose
instead there is no patent on wigitor, no intellectual property
protection. Think of this case as
representing India which has a history of not recognizing patents on
drugs. (As will be discussed below, it
has recently signed an agreement to recognize drug patents.) Or it is the United States, after the twenty
year term of the patent is up. With no
patent, firms producing generic versions of wigitor can freely compete with
Econland Big Pharma. Free entry and
competition will drive the price down to the marginal cost of $2. In this case, Econland Big Pharma makes zero
operating profit. (It may be that
through advertising, Econland Big Pharma might be able to obtain earn a slight
premium for the brand name version over the generic, but let’s ignore that here
to make things simple. The example of
Zucor above makes clear that the ability to extract profits falls precipitously
when drugs are off patent.)
Case C: Patent
Recognition and Price Regulation
Now
consider a third case where a country recognizes Econland Big Pharma’s patent,
but regulates the price it can set for the drug. To put it another way, it sits down with the
company and bargains with it. Canada is
an example. Suppose Canada bargains the
price down to $3. It’s bargaining stance
is that if Econland Big Pharma won’t sell wigitor for $3 or less, it will just
tell all the doctors in
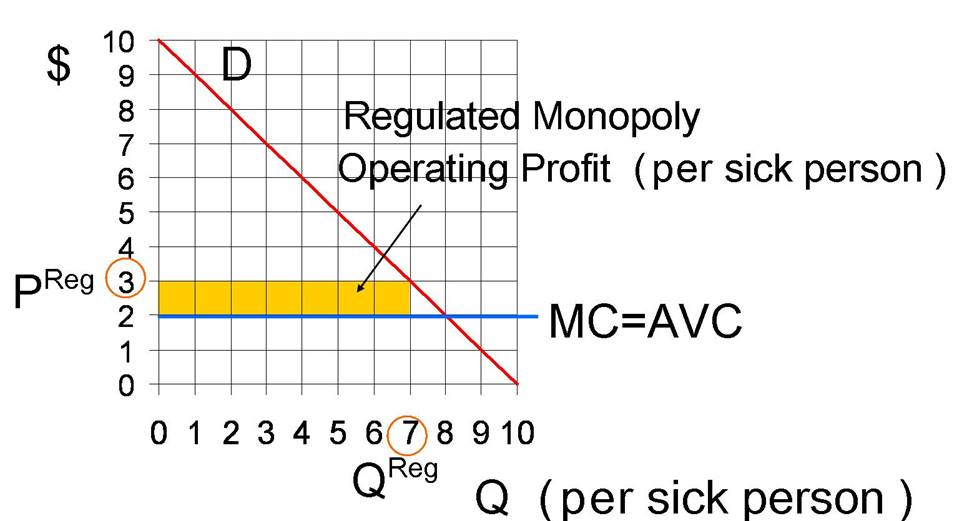
At
the regulated price of $3, the quantity is 7 units, and the operating profit is
$7=($3−$2)×7. The operating profit
is illustrated by the yellow rectangle in Figure 3.
Figure 3: Regulated
Monopoly
Let’s
put all of this together and examine the global operating profit Econland Big
Pharma can expect to receive if it develops wigitor. Let’s make the following additional assumptions:
·
The
demand per sick person described above is on an annual basis. If the firm gets a patent, it will be able to
make profits for 20 years while the patent is in force. To make things simple, we won’t take into
account the time value of money. (That a dollar twenty years from now is worth
less than a dollar today. Let’s put off
the concept of “present value” to another class.) So we will just multiply annual operating profits
by 20 to get operating profits for 20 years.
·
Assume
ten percent of the population is afflicted with the dreaded economyosis in any
given year.
·
United States: Econland Big Pharma will get a patent that
will last 20 years. There will be no
price regulation over this period. There
are 300 million people and this will remain constant over time. The demand per person remains constant over
time as graphed above. (In real world
applications, demand often shrinks towards the end of a patent’s life if new
and better substitute drugs are invented.)
·
Other Developed
Countries. There are 600 million people in other
developed countries like Canada and Germany.
These countries recognize the patent, but regulate prices to be no
higher than $3.
·
Rest of the World. The rest of the world does not recognize the
patent. Or even if they do, they are too
poor to buy drugs, so there is no money to be made from them in either case.
Let’s
put all of this together and calculate total operating profit over the lifetime
of the drug:
Annual Global Operating
Profit (in $ million)
= .1×300×16 (from
= $480 + $420 + 0
=$900 million per year.
To
understand the first term, observe that 10 percent of the 300 (million) people
in the U.S. get sick with economyosis in a given year and the operating profit
per year is $16 per sick person. This
delivers $480 million in annual operating profit from the U.S. The annual operating profit from the other
developed countries is a little smaller, $420 million, even though there are
twice as many people in these other countries.
This happens because these other countries regulate prices. Since the patent lasts for 20 years, we
multiple by 20 to get the operating profit over the lifetime of the drug,
Lifetime operating
profit: 20×$900 million = $18 billion.
Part 3: The Incentive
for Innovation
The
above analysis calculates the global operating profits to be had once wigitor
is invented. That is one important
factor entering into the decision of whether to invest in trying to develop the
drug. But it isn’t the only factor. Two other important considerations are:
First, the cost of the research and development (including costs of testing to
make sure the drug is safe and effective).
Second, the likelihood that the drug will be successful. The drug invention business is a risky one;
Many drugs turn out not to work, not to cure the intended disease. Or worse, the drugs might cause other
problems and have harmful side effects.
If it turns out that wigitor causes people to die of
politicalscienceosis, it will have to be pulled off the market (and numerous
lawsuits can be expected).
Suppose
that if Econland Big Pharma initiates an R&D project to create wigitor, the
likelihood of success (that it invents wigitor and that is cures economyosis
without harmful side effects) is 50 percent.
Since the project delivers $18 billion in operating profit if successful
and 0 otherwise, and since there is a 50/50 chance of the two events, the expected lifetime operating profit is
$9 billion (=.5×$18 billion). Define net
(lifetime) expected value of the project to be
Net Lifetime Expected
Value = Expected Lifetime Operating Profit − Fixed Cost of R&D
= $9 billion −
Fixed Cost of R&D
If
the fixed cost of research and development exceeds $9 billion dollars, then net
lifetime expected value is negative and Econland Big Pharma would certainly not
want to invest in this project. In
addition to being a bad deal in terms of expected value, there would be great
risk involved with the project. If the
research and development costs are less then $9 billion, then the project has
positive net expected value. The CBO
report mentioned above cites estimates of 800 million dollars for R&D fixed
costs for typical drug projects in recent years. I think it is quite likely that most big
pharmaceutical companies would be willing to pay $800 million dollars up front
in fixed R&D costs for a 50 percent chance at an $18 billion dollar payout
in operating profit.
Next
consider how a change in the extent
of intellectual property protection impacts the incentive to innovate. Suppose that instead of a 20 year patent
life, the law is changed so patents only last 5 years, a quarter as long. So now the operating profit if successful is
only .25×$18 billion = $4.5 billion. Taking into account that there is only a
50 percent chance of success, the expected operating profit is now only $2.25
billion. It is possible that Econland
Big Pharma might still choose to pursue this project. If so, it can be argued that it is a good
thing for society that the patent length was shortened, at least for this
case. We get the innovation either way
and the inefficiency of monopoly pricing is suffered for only 5 years rather
than 20 years. But if the fixed costs of
R&D are high enough that the company does not pursue the project under the
shorter patent (but would under the longer patent), then society is worse off
with the shorter patent. Wigitor is not
invented and society must live with the scourge of economyosis.
This
discussion highlights a tradeoff. If
patent protection is limited, there is less monopoly for a given amount of
innovation. (This increases total surplus).
But there is potentially less incentive to innovate. (This decreases total surplus.)
Part 4: International Agreements to Protect Intellectual Property in the Drug
Industry
As
noted above, drug companies typically generate little revenue from poor
countries for two reasons. Poor
countries can’t afford to pay much to begin with but on top of that, they
typically have ignored patents and have either produced (unlicensed) generic
equivalents themselves or purchased them from a country like India who produced
them. This is the same thing as buying a
knock-off CD or DVD.
It
isn’t that India didn’t recognize patents for anything. Rather, India’s 1970
patent law specifically excluded drugs from patent protection. India developed a large unlicensed generic
drug producing industry both for its own market and for other poor countries
(such as countries in Africa). One can
imagine India trying to motivate this exemption based on a moral case: That it
might be OK to patent a new kind of golf club but that it is “immoral” to
patent a life-saving drug. That may not
be the best logic: it is more important that there be incentives to create new
life-saving drugs than new golf clubs.
But this moral rhetoric is beside the point. It was in India’s self interest to ignore
drug patents. There was very little in
the way of any research and development in the drug industry taking place in
India to begin with. So these unlicensed
generic companies in India were not copying any Indian firms. Rather, they copied the innovations of
companies like Merck in developed countries.
In fact, India was better off that the developed countries did enforce patent laws in their own
countries. This created incentives for
companies like Merck to create new drugs that the Indian firms could then copy.
Naturally,
the big pharamceutical companies in the rich countries did not like this
situation and they lobbied to change it.
The opportunity to do something about it came from trade
agreements. The main agreement is called
Trade-Related Aspects of Intellectual Property Rights (TRIPS). See Barton (2004) for more information. The agreement dates from 1995 with subsequent
related agreements in 2001 and 2003.
Some of the main provisions came into effect in 2005. In this agreement, India and other developing
countries agreed to recognize drug patents.
They made this concession in return for trade concessions by rich
countries. In particular, that the rich
countries open their markets to goods like textiles from poor countries.
It
remains to see what will come of all this.
Poor countries like India are obviously not going to pay high prices for
drugs. So if drug companies want to sell
anything in poor countries, they will have to price discriminate and set very
low prices. In particular, with price
discounts much steeper than the ones Canada gets relative to the
In
2009, for example, India rejected patent applications for the HIV drugs
Tenofovir and Darunavir, which means the companies located in India that
produce generic equivalents of these drugs can continue to produce them. The plan is for these drugs to be exported
from India to Brazil, which has also rejected the patents. The Indian courts determined that these drugs
were not sufficiently new and novel compared to previous drugs and therefore
were undeserving of patent protection.
In contrast, in the U.S., the drugs were deemed sufficiently new and
novel to be granted patents.
Brazil
is also taking a tough line with the drug companies. As it is a medium income country, drug
companies want to charge it higher prices than poor countries pay. An Associated Press article
from May 2007 tells an interesting story about the HIV drug efavirenz. Brazil tried to bargain with Merck (the
producer of the drug) for a price of 65 cents per pill, the same price Thailand
paid. Merck argued that since Brazil was
richer than Thailand, it should pay $1.10 per pill, which was still a discount
down from the $1.57 per pill Merck was charging rich countries. After Brazil failed to get the 65 cents per
pill price, it decided to ignore the patent and instead buy “knock-off”,
unlicensed, generic equivalents of the drug.
Final Comments
Comment 1: For-profit drug companies like Merck
unsurprisingly direct R&D efforts towards drugs with the potential to
deliver high operating profits. These
are drugs that people who live in rich countries are going to use. So we see, for example, R&D investment
for many kinds of drugs to address erectile dysfunction. Merck has little incentive to invest in drugs
that are only used in poor countries because poor countries don’t want to pay
for them. (See above.) If such drugs are going to be developed,
someone has to be willing to pay for them.
The Bill and Melinda Gates
Foundation is an example of one such “someone.” For example, it is working to create new
vaccines for tuberculosis and has spent 750
million dollars to date on this project.
Comment 2: Part 3 above laid out
the standard economic argument for the tradeoffs involved when patent
protection is increased. For fixed levels
of innovation there is more monopoly.
But with more patent protection, there is more innovation. There is an important school of thought
challenging the notion that increasing patent production necessarily increases
innovation. In particular, Boldrin and
Levine (2008) make strong arguments that firms can use patents to block
innovations of rival firms. You can
check out their web site at “Against
Monopoly.”
Comment 3: For related issues involving technology to
prevent malnutrition, see this very interesting article “The
Peanut Solution,” New York Times Magazine, September 2, 2010.
References
Barton, John H. “Trips and the Global
Pharmaceutical Market,” Health Affairs, 23, no. 3 (2004): 146-154. Web
link
Boldrin, Michele and David K. Levine, “Against
Intellectual Monopoly,” Cambridge University Press: New York, 2008. Web
link
Butler, Declan, “India says No to HIV drug
patents,” Published online 3 September 2009, Nature New
doi:10.1038/news.2009.882 Web
link
Congressional Budget Office, Research and
Development in the Pharmaceutical Industry,” October 2006. Web
link